有機分子触媒を用いる不斉反応の開発と生理活性化合物合成への応用
メンバー: 長澤和夫、小田木陽
分野: 基礎化学、複合化学、生体分子科学
所属: 工学研究院
キーワード: 有機分子触媒、不斉合成、生理活性化合物、環境調和、天然物、全合成
ウェブサイト:
研究概要
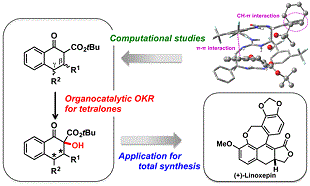
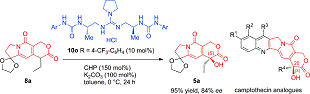
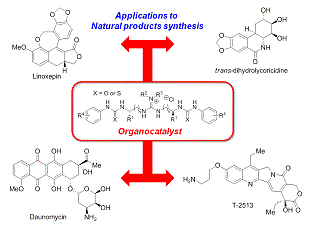
有機触媒は、環境調和型の触媒として、近年非常に注目されています。当研究室では、新規有機触媒の開発、開発した触媒を用いた不斉反応や新規反応の開発を行っています。また開発した有機触媒反応を基盤とした、有用生理活性物質の効率的な合成手法の開発も行っています。これまでに、抗がん剤として用いられている、ダウノマイシンやカンプトテシン類の新規合成法の開発にも成功しました。また次世代の抗がん剤候補化合物(リード化合物)の合成にも成功しています。環境に配慮した創薬研究に取り組んでいます。
(1)精密有機化学による機能性低分子化合物(有機分子触媒)の創出とそれを用いる新規反応開発
(2)有機分子触媒反応を鍵工程とする生理活性化合物(薬剤リード)の合成研究
(2)触媒反応のメカニズム解析と触媒コンセプトの体系化
主要論文・参考事項
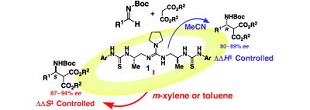
"Origin of stereocontorol in guanidine-bisurea bifunctional organocatalyst that promotes α-hydroxyaltion of tetralone-derived β-ketoesters: Asymmetric synthesis of β- or γ-substituted tetralone derivatives via organocatalytic oxidative kinetic resolution"
Minami Odagi, Kota Furukori, Yoshiharu Yamamoto, Makoto Sato, Keisuke Iida, Masahiro Yamanaka, and Kazuo Nagasawa
J. Am. Chem. Soc. 2015, 137, 1909-1915.
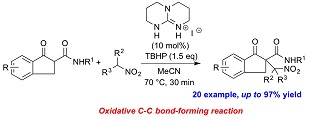
"Guanidium Iodine-Catalyzed Oxidative α-Nitroalkylation of β-Ketoamides"
Koji Yasui, Takanari Kato, Kohei Kojima, and Kazuo Nagasawa
Chem. Commun. 2015, 51, 2290-2293.
"Solvent-Dependent Enantiodivergent Mannich-Type Reaction: Utilizing a Conformationally Flexible Guanidine/Bisthiourea Organocatalyst"
Yoshihiro Sohtome,. Shinji Tanaka, Keisuke Takada, Takahisa Yamaguchi, and Kazuo Nagasawa
Angew. Chem. Int. Ed. 2010, 49, 9254-9257.
"Entropy-Controlled Catalytic Asymmetric 1,4-Type Friedel–Crafts Reaction of Phenols Using Conformationally Flexible Guanidine/Bisthiourea Organocatalyst"
Yoshihiro Sohtome, Bongki Shin, Natsuko Horitsugi, Rika Takagi, Keiichi Noguchi, and Kazuo Nagasawa
Angew. Chem. Int. Ed. 2010, 49, 7299-7303.
お問い合わせ先
東京農工大学・先端産学連携研究推進センター
urac[at]ml.tuat.ac.jp([at]を@に変換してください)
Development of organocatalytic asymmetric reactions and its application to synthesis of biologically active compounds

Research members: Kazuo Nagasawa PhD., Minami Odagi PhD.
Research fields: Basic chemistry, Applied chemistry, Biomolecular science
Departments: Institute of Engineering
Keywords: organocatalyst, asymmetric synthesis, biologically active compound, environmentally benign, natural product, total synthesis
Web site:
Summary

In our group, we are currently focusing on the development of environmentally friendly catalysis, i.e., organocatalysis. So far, varieties of asymmetric reactions as well as unique carbon-carbon bond- forming reactions have been developed on the basis of our new organocatalysis. Moreover, biologically active molecules of anti-cancer agents have been accomplished based upon our organocatalysis. Recently, natural products with potent biological activities with some enzyme inhibitory activities have also synthesized with the catalysis. We will keep pursuing the environmentally benign, efficient, and practical method for the synthesis of drug lead with organocatalysis.
(1) Development of new organocatalysis.
(2) Synthesis of biologically active molecules based upon the organocatalysis.
(3) Mechanistic studies of the organocatalytic reactions.
Reference articles and patents
"Origin of stereocontorol in guanidine-bisurea bifunctional organocatalyst that promotes α-hydroxyaltion of tetralone-derived β-ketoesters: Asymmetric synthesis of β- or γ-substituted tetralone derivatives via organocatalytic oxidative kinetic resolution"
Minami Odagi, Kota Furukori, Yoshiharu Yamamoto, Makoto Sato, Keisuke Iida, Masahiro Yamanaka, and Kazuo Nagasawa
J. Am. Chem. Soc. 2015, 137, 1909-1915.
"Guanidium Iodine-Catalyzed Oxidative α-Nitroalkylation of β-Ketoamides"
Koji Yasui, Takanari Kato, Kohei Kojima, and Kazuo Nagasawa
Chem. Commun. 2015, 51, 2290-2293.
"Solvent-Dependent Enantiodivergent Mannich-Type Reaction: Utilizing a Conformationally Flexible Guanidine/Bisthiourea Organocatalyst"
Yoshihiro Sohtome,. Shinji Tanaka, Keisuke Takada, Takahisa Yamaguchi, and Kazuo Nagasawa
Angew. Chem. Int. Ed. 2010, 49, 9254-9257.
"Entropy-Controlled Catalytic Asymmetric 1,4-Type Friedel–Crafts Reaction of Phenols Using Conformationally Flexible Guanidine/Bisthiourea Organocatalyst"
Yoshihiro Sohtome, Bongki Shin, Natsuko Horitsugi, Rika Takagi, Keiichi Noguchi, and Kazuo Nagasawa
Angew. Chem. Int. Ed. 2010, 49, 7299-7303.
Contact
University Research Administration Center(URAC),
Tokyo University of Agriculture andTechnology
urac[at]ml.tuat.ac.jp
(Please replace [at] with @.)