糖に作用するタンパク質の性質および構造の解析
メンバー: 西河淳、殿塚隆史
分野: 境界農学、生物科学、農芸化学
所属: 農学研究院
キーワード: 糖、タンパク質、酵素、がん、レクチン、糖鎖生物学、グリコシダーゼ
ウェブサイト:
研究概要

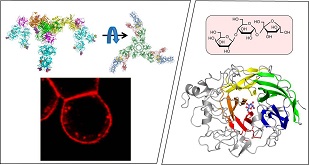
糖は、生体のエネルギー源、生体の構造維持、生体分子同士の認識など、さまざまな機能を持っている。糖は、構成糖の種類や結合様式が少し変わるだけで、その機能は劇的に変化する。糖のわずかな違いを検出することは、疾患の診断への応用につながる。また、澱粉のような糖から複雑な機能を持つ糖に変換する技術は、食品産業などへの応用が期待される。私達のグループは、糖に作用するタンパク質を材料とした研究を行っている。動物の特定の糖鎖に結合し侵入するタンパク質について、どの部位が糖の認識に関与しているのか明らかにしてきた(図左)。現在、本タンパク質を利用した糖検出技術の開発を行っている。また、機能性を有する糖を生産する酵素について立体構造を決定し、耐熱化酵素の作製を行った(図右)。このような研究をもとに、有用な糖の創出や酵素機能の改良を目指している。
主要論文・参考事項
1) Miyazaki, T., Ishizaki. Y., Ichikawa. M., Nishikawa. A., and Tonozuka, T. Structural and biochemical characterization of novel bacterial α-galactosidases belonging to glycoside hydrolase family 31. Biochem. J., 469, 145-158, 2015.
2) Iwasa, C., Tonozuka, T., Shinoda, M., Sagane, Y., Niwa, K., Watanabe, T., Yoshida, H., Kamitori, S., Takao, T., Oguma, K. and Nishikawa, A., Purification, crystallization and preliminary X-ray analysis of an HA17–HA70 (HA2–HA3) complex from Clostridium botulinum type C progenitor toxin. Acta Crystallogr. Sect. F, 70, 64-67, 2014.
3) Tonozuka, T., Tamaki, A., Yokoi, G., Miyazaki, T., Ichikawa, M., Nishikawa, A., Ohta, Y., Hidaka, Y., Katayama, K., Hatada, Y., Ito, T. and Fujita, K., Crystal structure of a lactosucrose-producing enzyme, Arthrobacter sp. K-1 β-fructofuranosidase, Enzyme Microb. Technol., 51, 359-365, 2012.
4) Nakamura, T., Tonozuka, T., Ito, S., Takeda, Y., Sato, R., Matsuo, I., Ito, Y., Oguma, K. and Nishikawa A., Molecular diversity of the two sugar-binding sites of the β-trefoil lectin HA33/C (HA1) from Clostridium botulinum type C neurotoxin. Arch. Biochem. Biophys., 512, 69-77, 2011.
お問い合わせ先
東京農工大学・先端産学連携研究推進センター
urac[at]ml.tuat.ac.jp([at]を@に変換してください)
Analysis of biochemical properties and structures of carbohydrate-acting proteins

Research members: Dr. Atsushi Nishikawa, Dr. Takashi Tonozuka
Research fields: Boundary agriculture, Biological Science, Agricultural chemistry
Departments: Institute of Agriculture
Keywords: carbohydrate, protein, enzyme, cancer, lectin, glycobiology, glycosidase
Web site:
Summary

Carbohydrates have various functions such as energy source for organisms, formation and maintenance of protein structures, and recognition and interaction between molecules. Functions of carbohydrates are drastically changed by subtle differences of monosaccharide units and glycosidic linkages. Detection of small differences of carbohydrates will allow us to develop a novel diagnosis system. Also, conversion of sugars like starch into functionally complicated carbohydrates will be useful in food and related industries. We are focusing on studies of carbohydrate-acting proteins. A protein that specifically binds and internalizes into mammal cells has been analyzed, and the results indicate which part of the protein is critical for the binding ability (left panel). We are now developing a new carbohydrate-detection system using this protein. Also, the structure of a functional sugar-producing enzyme has been determined, and some thermostabilized enzymes have been constructed (right panel). Our goal is to establish the production of useful carbohydrates and the manipulation of enzyme properties.
Reference articles and patents
1) Miyazaki, T., Ishizaki. Y., Ichikawa. M., Nishikawa. A., and Tonozuka, T. Structural and biochemical characterization of novel bacterial α-galactosidases belonging to glycoside hydrolase family 31. Biochem. J., 469, 145-158, 2015.
2) Iwasa, C., Tonozuka, T., Shinoda, M., Sagane, Y., Niwa, K., Watanabe, T., Yoshida, H., Kamitori, S., Takao, T., Oguma, K. and Nishikawa, A., Purification, crystallization and preliminary X-ray analysis of an HA17–HA70 (HA2–HA3) complex from Clostridium botulinum type C progenitor toxin. Acta Crystallogr. Sect. F, 70, 64-67, 2014.
3) Tonozuka, T., Tamaki, A., Yokoi, G., Miyazaki, T., Ichikawa, M., Nishikawa, A., Ohta, Y., Hidaka, Y., Katayama, K., Hatada, Y., Ito, T. and Fujita, K., Crystal structure of a lactosucrose-producing enzyme, Arthrobacter sp. K-1 β-fructofuranosidase, Enzyme Microb. Technol., 51, 359-365, 2012.
4) Nakamura, T., Tonozuka, T., Ito, S., Takeda, Y., Sato, R., Matsuo, I., Ito, Y., Oguma, K. and Nishikawa A., Molecular diversity of the two sugar-binding sites of the β-trefoil lectin HA33/C (HA1) from Clostridium botulinum type C neurotoxin. Arch. Biochem. Biophys., 512, 69-77, 2011.
Contact
University Research Administration Center(URAC),
Tokyo University of Agriculture andTechnology
urac[at]ml.tuat.ac.jp
(Please replace [at] with @.)

