Development of efficient synthetic methods of heterocyclic compounds
Research members: Akio Saito PhD.
Research fields: Basic chemistry, Pharmacy
Departments: Division of Applied Chemistry, Institute of Engineering
Keywords: heterocycles, catalysis, consecutive reaction, multi-component reaction, iodine
Web site:
Summary
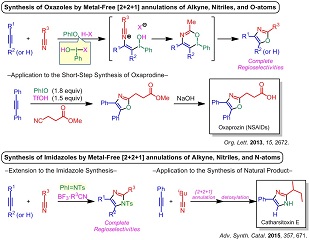
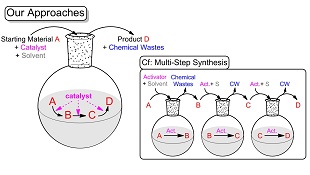
Resource-saving technologies are required in the modern society and thus the development of novel and effective procedures for syntheses of useful organic molecules is a crucial issue. From these viewpoints, considering "the effective utilization of carbon resources" and "reduction of chemical wastes", we have developed synthetic methods of heterocycle compounds, which are prevalent in a wide variety of useful compounds (such as medicines, agrichemicals, functional materials and so on), based on catalytic consecutive reactions and multi-component reactions. Since the catalytic system for the catalytic consecutive reactions and multi-component reactions activates plural reactions in one flask, these reactions provides us with not only "simpleness and easiness of operation" but also "improvement of the atom effectiveness" and "reduction of chemical wastes". Recently, we have mainly researched methods with iodine reagents because Japan is abundant in iodine. The following examples are some of our synthetic methods.
1) Syntheses of oxazoles and imidazoles based on [2+2+1]annulations of alkynes, nitriles and heteroatoms mediated by hypervalent iodine reagent as "a activating agent" and "a heteroatom donor"
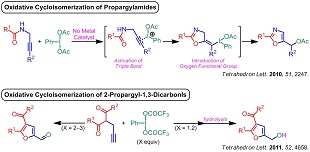
2) Syntheses of oxazoles and furans concomitantly with incorporation of oxygen functional groups based on the oxidative cycloisomerization of alkynes mediated by hypervalent iodine reagent as "an activating agent" and "a heteroatom donor"
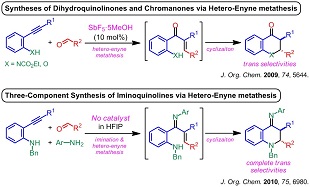
3) Syntheses of dihydroquinolinones and iminoquinolines based on the hetero-enyne metathesis reaction
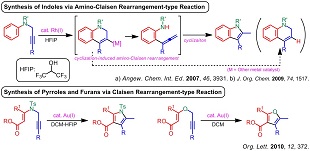
4) Syntheses of indoles and pyrroles based on the amino-Claisen rearrangement-type reaction
Reference articles and patents
(1) "Metal-Free [2+2+1]Annulation of Alkynes, Nitriles and N-Atoms from Iminoiodanes for Synthesis of Highly Substituted Imidazoles", A. Saito, Y. Kambara, T. Yagyu, K. Noguchi, A. Yoshimura, V. V. Zhdankin, Adv. Synth. Catal. 2015, 357, 667-671.
(2) "Metal-Free [2+2+1]Annulation of Alkynes, Nitriles, and Oxygen Atoms: Iodine(III)-Mediated Synthesis of Highly Substituted Oxazoles", A. Saito, A. Taniguchi, Y. Kambara, Y. Hanzawa, Org. Lett. 2013, 15, 2672–2675.
(3) "PIDA-Mediated Synthesis of Oxazoles through Oxidative Cycloisomerization of Propargylamides", A. Saito, A. Matsumoto, Y. Hanzawa, Tetrahedron. Lett. 2010, 51, 2247–2250.
(4) "Synthesis of Pyrroles by Gold(I)-Catalyzed Amino−Claisen Rearrangement of N-Propargyl Enaminone Derivatives", A. Saito, T. Konishi, Y. Hanzawa, Org. Lett. 2010, 12, 372-374.
(5) "Tandem Synthesis of 2,3-Dihydro-4-iminoquinolines via Three-Component Alkyne-Imine Metathesis", A. Saito, J. Kasai, T. Konishi, Y. Hanzawa, J. Org. Chem. 2010, 75, 6980–6982.
(6) "アルキン類の触媒的逐次反応を基盤とする複素環合成法の開発", 齊藤亜紀夫, 榛澤雄二, 有機合成化学協会誌, 2014, 72, 246–256. (account)
Contact
University Research Administration Center(URAC),
Tokyo University of Agriculture andTechnology
urac[at]ml.tuat.ac.jp
(Please replace [at] with @.)