Vacuolar enzymes of glycosyltransfer and acyltransfer for anthocyanins in plants
Research members: Dr. Yoshihiro Ozeki
Research fields: Basic biology, Integrated engineering, Agricultural chemistry
Departments: Institute of Engineering
Keywords: modification enzymes in plant vacuole, glycosyltransferase, acyltransferase, secondary metabolites, plant pigments, anthocyanin
Web site:
Summary

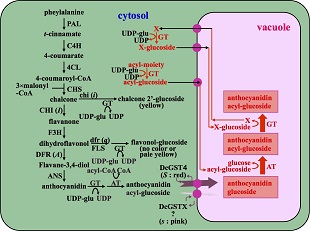
Plant flowers present us various varieties of colors. The major pigments of plant flowers are anthocyanins. While anthocyanins are consisted of six aglycones named as anthocyanidins, anthocyanidins are modified with various glycosyl and acyl residues to generate varieties of anthocyanin molecules showing various colors. The synthesis of aglycones and their modification with sugars and organic acids are thought to be catalyzed by cytosolic enzymes, the genes for which were isolated and introduced into rose and carnation to generate blue flowers; the transgenic rose and carnation could bear violet but not clear blue flowers. Some of modification enzymes for anthocyanin has not unknown, especially glucosyltransferase at 7 position of anthocyanidin should be elucidated in many plant species bearing blue flowers, which is required to shift anthocyanin color from red to blue hue. We found that, in blue flowers of delphinium, the glucosylation and acylation at 7 position of anthocyanin occurs not in cytosol but in vacuole, which is thought to be a static organelle to accumulate wastes of cytosolic metabolites. The genes for modification enzymes with glucosyl and acyl residues in vacuoles are identified from not only dicot plants including delphinium but also monocot plants such as agapanthus. It is expected that introduction of the genes for these enzymes will give us transgenic plants bearing clear blue flowers to plants bearing red and purple flowers such as rose and carnation.
Reference articles and patents
Miyahara, T., Tani, T., Takahashi, M., Nishizaki, Y., Ozeki, Y. and Sasaki, N. Isolation of anthocyanin 7-O-glucosyltransferase from Canterbury bells (Campanula medium). Plant Biotechnol. 31(5):555-559 (DOI: 10.5511/plantbiotechnology.14.0908a) (2014).
Nishizaki, Y., Sasaki, N., Yasunaga, M., Miyahara, T., Okamoto, E., Okamoto, M., Hirose, Y. and Ozeki, Y. Identification of the glucosyltransferase gene that supplies the p-hydroxybenzoyl-glucose for 7-polyacylation of anthocyanin in delphinium. J. Exp. Bot., 65(9): 2495-2506 (doi:10.1093/jxb/eru134) (2014).
Yagi, M., Kosugi, S., Hirakawa, H., Ohmiya, A., Tanase, K., Harada, T., Kishimoto, K., Nakayama, M., Ichimura, K., Onozaki, T., Yamaguchi, H., Sasaki, N., Miyahara, T., Nishizaki, Y., Ozeki, Y., Nakamura, N., Suzuki, T., Tanaka, Y., Sato, S., Shirasawa, K., Isobe, S., Miyamura, Y., Watanabe, A., Nakayama, S., Kishida, Y., Kohara, M., and Tabata, S. Sequence analysis of the genome of carnation (Dianthus caryophyllus L.). DNA Res. 21(2): 231-241 (DOI:10.1093/dnares/dst053) (2014).
Nishizaki, Y., Yasunaga, M., Okamoto, E., Okamoto, M., Hirose, Y., Yamaguchi, M., Ozeki, Y. and Sasaski, N. p-Hydroxybenzoyl-glucose is a “Zwitter donor” for the biosynthesis of polyacyl-anthocyanin in delphinium. Plant Cell, 25(10): 4150-4165 (2013) (DOI: 10.1105/tpc.113.113167).
Nishizaki, Y., Matsuba, Y., Okamoto, E., Okamura, M., Ozeki, Y. and Sasaki, N. Structure of the acyl-glucose-dependent anthocyanin 5-O-glucosyltransferase gene in carnations and its disruption by transposable elements in some varieties. Mol. Gen. Genomics, 286(3): 383-394 (2011) (DOI: 10.1007/s00438-011-0655-7).
Matsuba, Y., Sasaki, N., Tera, M., Okamura, M., Abe, Y., Okamoto, E., Nakamura, H., Funabashi, H., Saito, M., Matsuoka, H., Nagasawa, K and Ozeki, Y. A novel glucosylation reaction on anthocyanins catalyzed by acyl-glucose dependent glucosyltransferase in the petals of carnation and delphinium. Plant Cell, 22(10): 3374-3389 (2010) (DOI: 10.1105/tpc.110.077487).
Contact
University Research Administration Center(URAC),
Tokyo University of Agriculture andTechnology
urac[at]ml.tuat.ac.jp
(Please replace [at] with @.)
